FACTS, FIGURES AND
BACKGROUND INFORMATION
HIGH REQUIREMENT PROFILE FOR LABORATORIES
Only laboratories possessing QS approval may be commissioned to carry out tests within the fra-
mework of QS feed monitoring. In order to obtain this approval, laboratories must be accredited in
accordance with DIN EN ISO/IEC 17025. They must also provide proof that they participated in round-
robin tests on the relevant parameters within the last twelve months prior to recognition. In addition,
QS ensures that all laboratories use the stipulated testing methods and requires a list of parameters
and their determination limits as well as analysis margins for the feed sector. In order to maintain
their QS recognition, all laboratories must participate in regular mandatory laboratory competence
tests.
SPECIALISED KNOWLEDGE FOR SAMPLING
All business that produce or trade in feed must participate in feed monitoring. The participants can
take the samples for this purpose themselves. Although this may not appear to be a neutral process
at first glance, it nevertheless makes for a high level of safety due to the cross-stage approach in
the QS scheme – because each stage takes samples from both incoming raw materials and outgoing
goods. This creates a system of mutual control within the chain. In the agricultural sector, on the
other hand, the sampling process is organised by the coordinators; samples must always be taken
in agricultural businesses by third parties, and the feed samples are mainly taken by the auditors
during the independent inspections. The basic rule is that only qualified personnel may take sam-
ples.
RISK-BASED CONTROL PLANS
There are a high number of different control plans within the framework of QS feed monitoring, and
these plans are tailored to the specific requirements of the sector in question. The control plans are
regularly reviewed and can be adapted as soon as it becomes necessary to react to topical deve-
lopments and occurrences in the market. The test results are naturally also taken into account when
preparing the control plans. If products have a conspicuous number of positive findings, then the
testing frequency is increased. By the same token, testing frequency can be reduced if numerous
tests show a low risk. Since 2014, for example, breweries no longer have to test their by-products
for salmonella as often as they used to.
■
REPORTING OBLIGATION IN THE CASE OF INCIDENTS
COMPARISON: OFFICIAL MONITORING
AND QS FEED MONITORING
Number of tested samples by business category
At livestock farmers 4,536 4,296 5,072 5,381
At producers or traders 10,668 10,294 11,499 11,996
Total 15,204 14,590 16,571 17,377
At livestock farmers 4,674 4,547 5,057 4,999
At producers or traders 21,045 17,970 15,612 13,576
Total 25,719 22,517 20,669 18,575
Official annual
statistics*
QS monitoring
2013
2012
2011
2010
2012
2011
2013
2010
2013
2012
2011
2010
QS monitoring
Testing frequencies for undesirable substances
Undesired substances with
stipulated maximum level,
including:
39.299 38.667 36.762 35.088
Aflatoxin B1 2,287 1,815 1,811 1,810
Organic chlorine compounds
1
8,829 9,746 10,974 10,581
Heavy metals
2
12,170 11,870 11,272 10,878
Dioxins 3,577 3,365 3,225 2,396
Spread of coccidiostats 9,989 9,721 7,609 7,530
39,299 38,667 36,762 35,088
Undesired substances without
stipulated maximum level,
including:
13.939 13.462 13.474 11.426
PCBs 3,489 3,177 2,993 2,444
Mycotoxins (except aflatoxin B1) 7,587 7,117 7,486 7,233
Total 53,238 52,129 50,206 46,514
13,939 13,462 13,474 11,426
Undesired substances with
stipulated maximum level,
including:
52.525 41.280 33.169 24.902
Aflatoxin B1 5,049 2,495 2,361 2,205
Organic chlorine compounds 13,322 10,403 7,527 3,109
Heavy metals (Pb, Cd, Hg, Ca) 22,952 21,016 17,560 15,595
52,525 41,280 33,169 24,902
Undesired substances without
stipulated maximum level,
including:
15.689 14.136 10.114 9.212
PCB (non-dioxin-like PCBs) 3,654 2,960 411 169
Mycotoxins (DON, ZEA, OTA) 12,035 11,176 9,703 9,043
Total 68,214 55,416 43,283 34,114
15,689 14,136 10,114 9,212
Dioxins, dioxin-like PCBs and
total dioxins and dioxin-like
PCBs, of which:
11.202 7.366 5.721 3.993
– Dioxins 4,554 2,843 2,376 1,789
– PCB (dioxin-like PCBs) 4,379 2,681 2,053 1,644
– Total dioxins and dioxin-like
PCBs 2,269 1,842 1,292 560
11,202 7,366 5,721 3,993
Official annual
statistics*
*Excerpt from the results of official feed monitoring in Germany in the control year 2013, Federal Ministry of Food
and Agriculture.
N.B. Legally stipulated maximum levels came into force for the parameter “non-dioxin-like PCBs” on 18 April 2012,
but the parameter is still listed in this table under “Undesirable substances without stipulated maximum level”.
1
Chlordan, DDT, dieldrin, endosulfan, endrin, heptachlor, hexachlorbenzene,
α
- and
β
-HCH, gamma-HCH (lindane)
2
Lead, mercury, arsenic, cadmium
Imprint
Editor
QS Qualität und Sicherheit GmbH
Dr. Hermann-Josef Nienhoff, Managing Director
Schedestraße 1-3
53113 Bonn
Germany
Phone +49 (0)228 350 68-0
Fax +49 (0) 228 350 68-10
Pictures: QS Qualität und Sicherheit GmbH, Shutterstock
Data basis: Analysis results of QS feed monitoring
from January 2008 to September 2014
THE EDITOR: WHO IS QS?
Since 2001 the QS scheme provides for food safety - from farm to shop. Today
95 percent of the pork and poultry meat produced in Germany originates from QS-
certified farms, the share of beef is 70 percent. More than 79,000 livestock farmers
take part in the QS scheme. The common goal: Consistent self-assessment as well
as comprehensive process assurance and traceability. Producers of fresh fruit,
vegetables and potatoes are added. Within the QS scheme together they produce
safe food according to specific QS requirements, supported by all up- and downst-
ream stages of the food supply chain.
■
Values above the maximum level:
the batch must be blocked; the product is no longer suitable
for use. In addition, the scheme participant must report the matter to the feed monitoring authority
as well as QS head office using the paper of incident.
■
Values above the “intervention level”:
if values exceed the intervention level, the business must
carry out an in-depth investigation of its processes, establish the cause and initiate corrective ac-
tion. The product may remain in circulation, however. Reporting of the matter to QS head office
is mandatory. The feed monitoring authority should also be informed.
■
Values above the guidance value:
if values exceed the QS guidance value stipulated for selected
active substances and target animals (e.g. aflatoxin B1 for dairy cattle), the rule for scheme par-
ticipants is that the product is still legally fit for circulation but may no longer in all cases be sold
to QS scheme participants. The matter is to be reported to QS head office (QS paper of incident),
who coordinates the further procedure with the scheme participant.
■
The business must report all
positive findings
for salmonella, antibiotically active substances and
animal constituents to QS head office (QS paper of incident). Notification of the feed monitoring
authority is recommended. In addition, it is necessary to differentiate with regard to the serovar,
the antibiotically active substance or the animal species.
■
If values are measured for DON, ZEA or OTA that
exceed the EU reference value
, there is no obli-
gation to report the matter to QS. However, in-house measures for handling of the product must
be stipulated and documented.
Remark:
Besides the obligation to report such information to QS, in many cases it is also required
to report to the supervisory authorities.
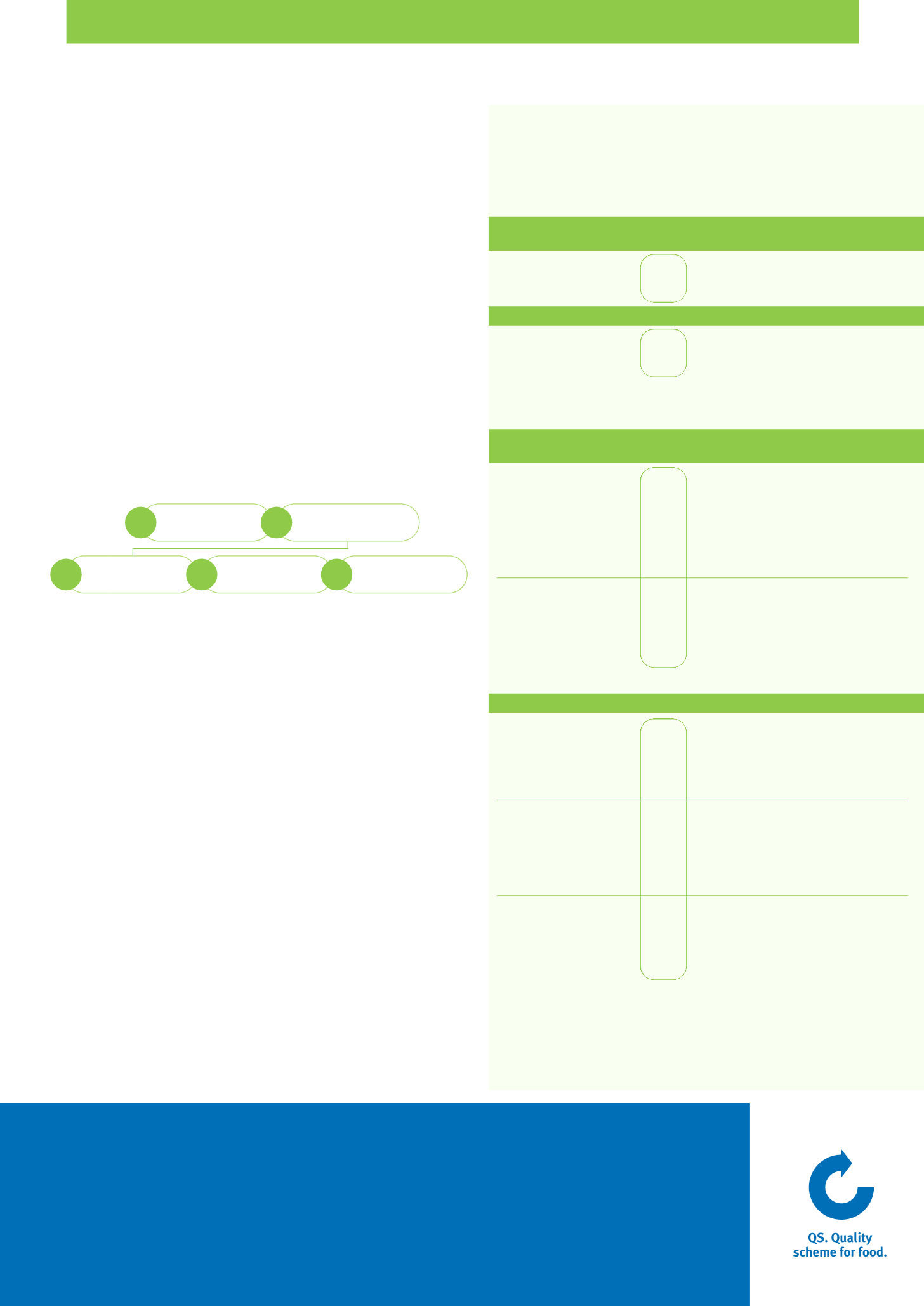
sample.
1.
dinator takes the
coor
oducer/trader/
The feed pr
in the QS database.
2.
.
analyses the sample.
4.
in the database.
5.
esults for the sample
test r
es the
y stor
The laborator
y
The laborator
y
he laborator
sample is sent
elated data
enters the sample r
dinator
coor
oducer/trader/
The pr
to t
3.
The
VON DER PROBENZIEHUNG BIS IN DIE QS-DATENBANK
2012
2011
2012
2011
2013
2010
Monitoring-
Report Feed 2014
As of September 2014


